
NCERT Exemplar Class 12 NCERT Exemplar Chemistry Solutions Chapter 2 Solutions Click to
Question 1. Calculate the mass percentage of benzene (C 6 H 6) and carbon tetrachloride (CCI 4) if 22 g of benzene is dissolved in 122 g of carbon tetrachloride. Solution: Mass of solution = Mass of benzene + Mass of carbon tetrachloride = 22 g +122 g = 144 g Mass percentage of benzene = ∴ Mass percentage of CCl 4

NCERT Exemplar Class 12 NCERT Exemplar Chemistry Solutions Chapter 2 Solutions Click to
Prime Members Can Enjoy Unlimited Free Shipping, Early Access To Lightning Deals and More. Choose From a Wide Selection Of Informative and Comprehensive Books For You.

NCERT Solutions for Class 12 Chemistry Chapter 2 Solutions
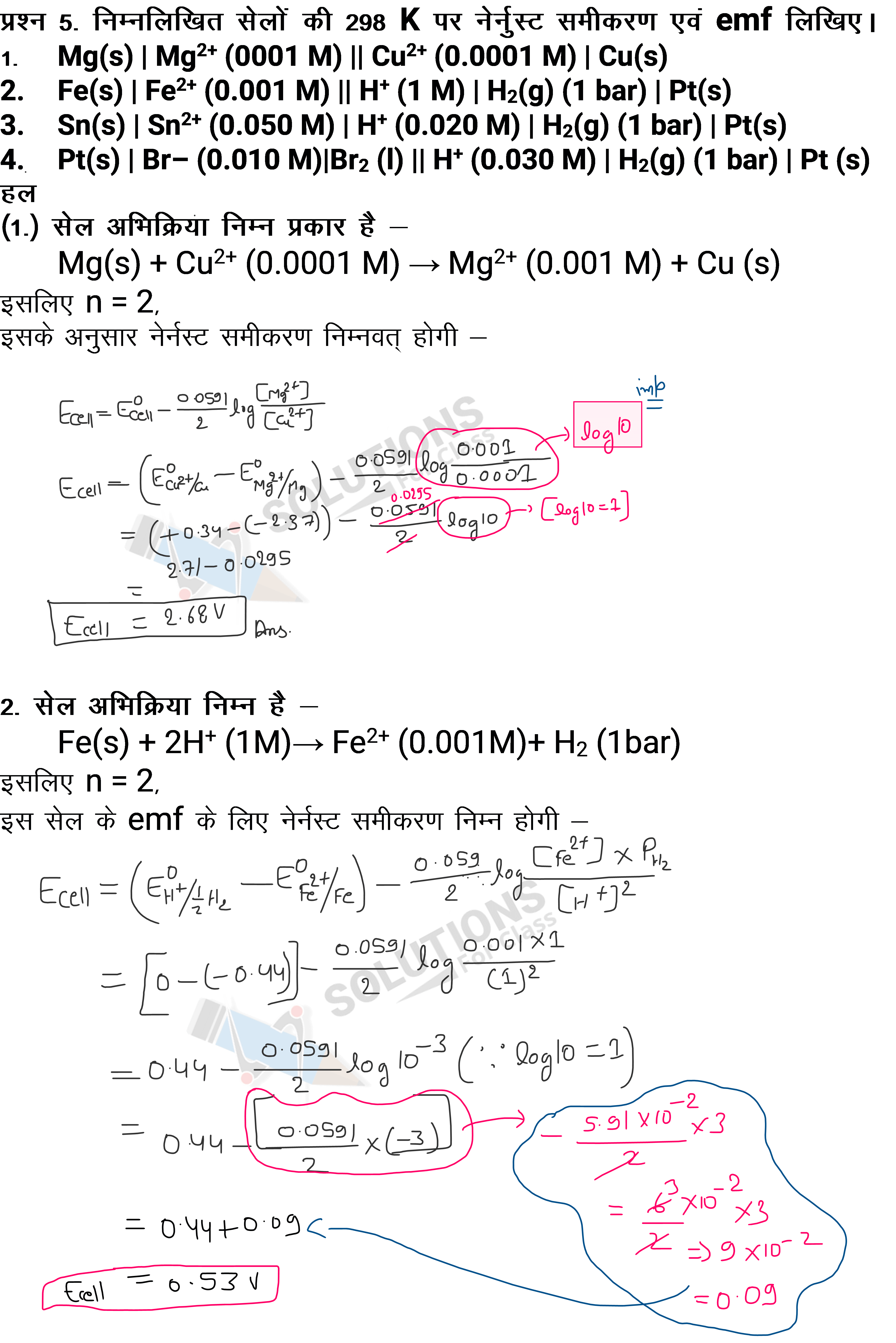
NCERT Solutions for Class 12 Chemistry Chapter 2 - Solutions = 0.12 mol Moles of water = = 4.44 mol Therefore, mole = = 0.0263 Q 2.6) Calculate Henry's law constant when the solubility of H 2 S (a toxic gas with a rotten egg-like smell) in water at STP is 0.195 m Answer 2.6: It is given that the solubility of H 2

NCERT Solutions for Class 12 Chemistry Chapter 2 in Hindi and English
NCERT solutions for Chemistry Class 12 Chapter 2 Solutions Intext Questions Intext Questions | Q 1 | Page 37 Calculate the mass percentage of benzene (C 6 H 6) and carbon tetrachloride (CCl 4) if 22 g of benzene is dissolved in 122 g of carbon tetrachloride. VIEW SOLUTION Intext Questions | Q 2 | Page 37

NCERT Exemplar Class 12 NCERT Exemplar Chemistry Solutions Chapter 2 Solutions Click to
NCERT Solutions for Class 12 Chemistry: Download Chapterwise PDFs. Chapter 1 The Solid State. Chapter 2 Solutions. Chapter 3 Electrochemistry. Chapter 4 Chemical Kinetics. Chapter 5 Surface Chemistry. Chapter 6 General Principles and Processes of Isolation of Elements. Chapter 7 The p-Block Elements.
Chemistry Class 12 Ncert Solutions Chapter 1 The Solid State Class 12 Gambaran
The Chemistry Class 12 NCERT Solutions Chapter 2 discusses several important concepts such as Raoult's Law, the types of solutions, the vapour pressure of liquid solutions, solubility of solids and gases in a liquid, ideal and non-ideal solutions, the concentration of solutions, colligative properties and determination of molar masses.
NCERT Solutions for Class 12 Chemistry Chapter 3 Electrochemistry (विधुत रसायन) Solutions For
Class 12 Chemistry NCERT Exemplar Solutions. Class 12 Chemistry NCERT Exemplar Solutions is about learning the formation of different types of solutions, Henry's law and Raoult's law, and the difference between non-ideal and ideal solutions. In the Class 12 CBSE board examination, questions are always asked from this chapter.

NCERT Solutions for Class 12 Chemistry Chapter 2 Solutions
The NCERT solutions for class 12 chemistry chapter 2: Solutions is given below in pdf as well as image form. NCERT Solutions for Class 12 Chemistry Chapter 2: Solutions - Important Topics A Solution is a homogeneous mixture of two or more chemically non-reacting substances whose composition can be varied within certain limits.

Download 1833 NCERT Solution Chemistry For Class12 by Subha Raghavan PDF Online
Chapter 1: Solutions Chapter 2: Electrochemistry Chapter 3: Chemical Kinetics Chapter 4: The d & f Block Elements Chapter 5: Coordination Compounds Chapter 6: Haloalkanes and Haloarenes Chapter 7: Alcohols, Phenols, and Ethers Chapter 8: Aldehydes, Ketones, and Carboxylic Acids Chapter 9: Amines Chapter 10: Biomolecules

NCERT Solutions for Class 12 Chemistry Chapter 15 Polymers
NCERT Solutions for Class 12 Chemistry Chapter 2 deals with Solutions. This chapter involves liquid solutions and their formations. Furthermore, the chapter involves the properties of the solutions. This chapter has information on types of solutions and their formation.

NCERT Solutions for Class 12 Chemistry Chapter 2 Solutions
Chapter 2 Solutions Chapter 3 Electrochemistry Chapter 4 Chemical Kinetics Chapter 5 Surface Chemistry Chapter 6 General Principles and Processes of Isolation of Elements Chapter 7 The p-Block Elements Chapter 8 d-and f-Block Elements Chapter 9 Coordination Compounds Chapter 10 Haloalkanes and Haloarenes Chapter 11 Alcohols, Phenols and Ethers

chemistry chapter 13 class 12 ncert solutions
NCERT solutions of class 12 Chemistry - Chapter List: • Chapter 1: Solid State. • Chapter 2: Solutions. • Chapter 3: Electrochemistry. • Chapter 4: Chemical Kinetics. • Chapter 5: Surface Chemistry. • Chapter 6: Principle Of Isolation Of Elements. • Chapter 7: P Block Elements. • Chapter 8: D And F Block Elements.

Solutions Short Notes Solution Chapter In Chemistry Class 12 Notes Hot Sex Picture
The NCERT Solutions for Class 12 Chemistry can be utilised by the students to prepare for the board examination and to solve the exercise questions of the Class 12 Chemistry Chapter 2. All the solutions are created according to the latest CBSE Syllabus for 2023-24 and its guidelines.

Ncert Solution For Class 12 Chemistry Chapter 3 Electrochemistry
Topics and Subtopics in NCERT Solutions for Class 12 Chemistry Chapter 2 Solutions: NCERT TEXTBOOK QUESTIONS SOLVED 2.1. Calculate the mass percentage of benzene (C6H6) and carbon tetrachloride (CCl4) if 22 g of benzene is dissolved in 122 g of carbon tetrachloride. Ans: Mass of solution = Mass of C 6 H 6 + Mass of CCl 4 = 22 g+122 g= 144 g

NCERT Solutions for Class 12 Chemistry Chapter 2 Solutions Free PDF
Class 12 Chemistry Chapter 2 Solutions are defined as homogeneous mixture of two or more components. This chapter gives an overview of different types of solutions. Various laws and its derivations are provided here stepwise for easy understanding.

Class 12th Chemistry Solutions NCERT Solution CBSE 2023
Students can download the CBSE Class 12 Chemistry NCERT Solutions PDF for free from Vedantu. Class 12 Chemistry teaches about organic, inorganic, and physical chemistry. It builds the base of applied science. Each chapter in these NCERT Solutions helps students to acquire in-depth knowledge of chemical compounds, polymers, biomolecules and.